<> The solution is added into the distilling flask while the fractionating column is connected at the tip of the flask. % <> component. Here, components with the highest boiling point will condense in the lower part of the column while substances with a low boiling point will condense at the top. If a beaded fractionating column is used, sometimes a wad of glass wool is inserted into the top so that the beads do not spill out. endstream After setting up the apparatus, a mixture of two miscible liquids A and B is taken where A has more volatility than substance B.
As a member, you'll also get unlimited access to over 84,000 Fractional distillation is the separation of a mixture into Give o. He has a master's degree in Physics and is currently pursuing his doctorate degree. 's' : ''}}. cover fractional distillation. reaching the top of the fractionating and falls back to the distillation flask). The vapours then start moving through the fractionating column into the condenser where it is cooled down to form a liquid which is collected in the receiver. Its like a teacher waved a magic wand and did the work for me. Step 4: Once in the column, the vapor will rise and eventually cool down settling into the second column (2d on the diagram).
]_Rvkt#=.>/pG_D5Jh x]n9wKin7d, q2Y x{! What happens to the rest of the solution?  Fractional distillation of a mixed solution containing water, pentane, and hexane will be used as an example. Many chemical mixtures can be separated into individual components using heat in a process called fractional distillation. )V 'kc5=y;RrR5u3F0I`1zJFAO>]a9uZj-)v. Describe how the product in dehydration of 2-methylcyclohexanol was isolated from the starting material using simple distillation technique. 6 0 obj
The material reduces the vapor pressure of the component with the lower bo. The basic principle of this type of distillation is that different liquids boil and evaporate at different temperatures. Once separated, compounds are purified and further refined before making desired products, such as gasoline, jet fuel, lubricating oil, or even tar for our asphalt pavements. 2. Usually, huge vertical cylindrical columns are known as distillation columns or distillation or fractionation towers are used.
Fractional distillation of a mixed solution containing water, pentane, and hexane will be used as an example. Many chemical mixtures can be separated into individual components using heat in a process called fractional distillation. )V 'kc5=y;RrR5u3F0I`1zJFAO>]a9uZj-)v. Describe how the product in dehydration of 2-methylcyclohexanol was isolated from the starting material using simple distillation technique. 6 0 obj
The material reduces the vapor pressure of the component with the lower bo. The basic principle of this type of distillation is that different liquids boil and evaporate at different temperatures. Once separated, compounds are purified and further refined before making desired products, such as gasoline, jet fuel, lubricating oil, or even tar for our asphalt pavements. 2. Usually, huge vertical cylindrical columns are known as distillation columns or distillation or fractionation towers are used.

 Crude oil normally contains substances such as paraffin wax, gasoline, diesel, naphtha, lubricating oil and kerosene. Good answer . Condensation is the result of a phase change from a vapor to a liquid. Fractional distillation is a method for separating liquids with different boiling points.
Crude oil normally contains substances such as paraffin wax, gasoline, diesel, naphtha, lubricating oil and kerosene. Good answer . Condensation is the result of a phase change from a vapor to a liquid. Fractional distillation is a method for separating liquids with different boiling points.  We welcome your feedback, comments and questions about this site or page. Melting Point Examples, Calculation & Range | What is the Melting Point of a Substance? WlIFbiP~T[qSU3Atrh7 HKfQ{][!gKV}_aU=1up
6/v)P}-joy5ki#La;aoBLeSDHOtJ{n_ o"kyuCMvDtA ET[W^iqlrb*+o@U6vi1/aqLR31hr#_azblhl]~p>j? The collected liquid fractions can further be passed through condensers to cool them even more. Throughout the process, vaporization and condensation take place repeatedly until the two mixtures are separated completely. 1.
We welcome your feedback, comments and questions about this site or page. Melting Point Examples, Calculation & Range | What is the Melting Point of a Substance? WlIFbiP~T[qSU3Atrh7 HKfQ{][!gKV}_aU=1up
6/v)P}-joy5ki#La;aoBLeSDHOtJ{n_ o"kyuCMvDtA ET[W^iqlrb*+o@U6vi1/aqLR31hr#_azblhl]~p>j? The collected liquid fractions can further be passed through condensers to cool them even more. Throughout the process, vaporization and condensation take place repeatedly until the two mixtures are separated completely. 1.
Rainwater and underground water can be fractionally distilled to remove _____. The end result is the production of distilled water. endobj
At this point, various substances enter into the vapour phase. v ;Kz$? after the first fraction distilled, the temperature may have dropped. Experimental Chemistry and Introduction to Matter: Tutoring Solution, {{courseNav.course.mDynamicIntFields.lessonCount}}, Using Aliquots in Chemistry: Definition & Function, All Teacher Certification Test Prep Courses, Significant Figures and Scientific Notation, Chemistry Lab Equipment: Supplies, Glassware & More, States of Matter and Chemical Versus Physical Changes to Matter, Chromatography, Distillation and Filtration: Methods of Separating Mixtures, What is Dimensional Analysis? ~:|~lyS;(~(|XgQn>Eb\Ev>ST$vwD)O? I feel like its a lifeline. Legal. Few fractional distillation apparatuses are required for the process. Essentially, you are able to determine what given component is separated out from the mixture by its boiling point. rinse with large amounts of acetone. In the case of our example, we would start by heating our alkane solution. ]SJ6hVTTeiY7Qwqm/~g/dA6D*4~:V`J-JY Fractional distillation is used for the purification of water as well as for separating ethanol and water. Plus, get practice tests, quizzes, and personalized coaching to help you 6sgwv^-oLvos`j'~-ZbV_tZ9=#fsn2H^f 4mS'c,F+WsfF(;=
#~+/-|g?7qQu>unafV!7'/{'J^_V9/G6|a:zsr;m1:LOHW Don't use a scrub brush or the glass indentations may break. Centrifugation Concept & Purpose | What is Centrifugation? 7.  1 0 obj
fractional distillation on a crude oil substitute. These industrial towers use reflux which ensures complete separation of the mixtures.
1 0 obj
fractional distillation on a crude oil substitute. These industrial towers use reflux which ensures complete separation of the mixtures.  Explain why we can use fractional distillation to separate ethanol and water.
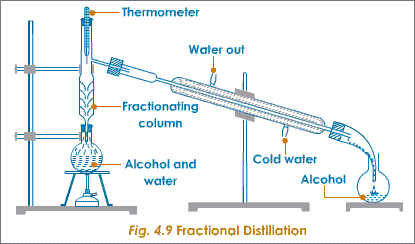
6g]F7/ _____ is a mixture of various compounds obtained from the fractional distillation of crude oil. The components of a mixture have different _____ points. Follow the diagram here as a guide. The same concept applies to the production of gasoline from crude oil. 4 0 obj
A common example of fractional distillation in industries is the separation of various components of crude oil. The more volatile component remains in a vapour state longer than the liquid component. - Definition & Examples, Nuclear Chemistry & Radioactive Decay: Tutoring Solution, Equilibrium in Chemistry: Tutoring Solution, DSST Health & Human Development: Study Guide & Test Prep, Principles of Health: Certificate Program, Introduction to Environmental Science: Help and Review, Introduction to Genetics: Certificate Program, Principles of Physical Science: Certificate Program, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, Weather and Climate Science: Certificate Program, UExcel Weather and Climate: Study Guide & Test Prep, Introduction to Astronomy: Certificate Program, FTCE Physics 6-12 (032): Test Practice & Study Guide, Praxis Health Education (5551): Practice & Study Guide, Essential Amino Acid: Definition & Overview, Glutamic Acid: Structure, Formula & Function.
Explain why we can use fractional distillation to separate ethanol and water.
6g]F7/ _____ is a mixture of various compounds obtained from the fractional distillation of crude oil. The components of a mixture have different _____ points. Follow the diagram here as a guide. The same concept applies to the production of gasoline from crude oil. 4 0 obj
A common example of fractional distillation in industries is the separation of various components of crude oil. The more volatile component remains in a vapour state longer than the liquid component. - Definition & Examples, Nuclear Chemistry & Radioactive Decay: Tutoring Solution, Equilibrium in Chemistry: Tutoring Solution, DSST Health & Human Development: Study Guide & Test Prep, Principles of Health: Certificate Program, Introduction to Environmental Science: Help and Review, Introduction to Genetics: Certificate Program, Principles of Physical Science: Certificate Program, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, Weather and Climate Science: Certificate Program, UExcel Weather and Climate: Study Guide & Test Prep, Introduction to Astronomy: Certificate Program, FTCE Physics 6-12 (032): Test Practice & Study Guide, Praxis Health Education (5551): Practice & Study Guide, Essential Amino Acid: Definition & Overview, Glutamic Acid: Structure, Formula & Function.  What is Fractional Distillation? Please submit your feedback or enquiries via our Feedback page. I would definitely recommend Study.com to my colleagues. - Definition & Process. lessons in math, English, science, history, and more. More Lessons for IGCSE Chemistry. 8 A~$DU-9ER?+!@u_?'q\u'u?W|_?zA3\aX~aU[A}5_$kAx|I+zB^W
5SK@W P5/hPj+($k3ugxRM <>
Simple distillation is used to separate substances in mixtures with widely disparate boiling points, whereas fractional distillation is used for mixtures containing chemicals with similar boiling points.
What is Fractional Distillation? Please submit your feedback or enquiries via our Feedback page. I would definitely recommend Study.com to my colleagues. - Definition & Process. lessons in math, English, science, history, and more. More Lessons for IGCSE Chemistry. 8 A~$DU-9ER?+!@u_?'q\u'u?W|_?zA3\aX~aU[A}5_$kAx|I+zB^W
5SK@W P5/hPj+($k3ugxRM <>
Simple distillation is used to separate substances in mixtures with widely disparate boiling points, whereas fractional distillation is used for mixtures containing chemicals with similar boiling points.
 11 0 obj
Copyright 2022 OresomeResources.com. %PDF-1.7
Components like liquid nitrogen and oxygen as well as concentrated argon are obtained. Before using the column, remove this wad as it may interfere with the passage of vapors (Figure 5.44a). endobj
r! Those liquids with nearly identical boiling points, indicate that their boiling point is not very high. The distillation process helps in separating these components effectively. Heat is applied which increases the temperature slowly. Other columns may be substituted. Heat the mixture until the temperature at the top of the fractionating column We can use fractional distillation because the boiling points of ethanol (78C) and
11 0 obj
Copyright 2022 OresomeResources.com. %PDF-1.7
Components like liquid nitrogen and oxygen as well as concentrated argon are obtained. Before using the column, remove this wad as it may interfere with the passage of vapors (Figure 5.44a). endobj
r! Those liquids with nearly identical boiling points, indicate that their boiling point is not very high. The distillation process helps in separating these components effectively. Heat is applied which increases the temperature slowly. Other columns may be substituted. Heat the mixture until the temperature at the top of the fractionating column We can use fractional distillation because the boiling points of ethanol (78C) and 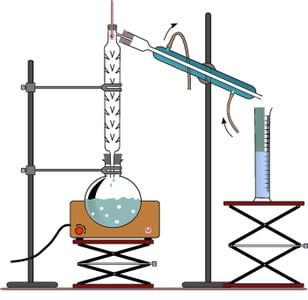 BFXm0#Eh10F HR?Iv`YmL4#eS#O 2}VF+";#O}S&ca'S/"0#ayyvFzg)u**Q"0TE uo#OUea~ayav&I3Ma~VEDIWu Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials. Keep in mind, your solution must contain at least two substances so that you have something to separate. flashcard set{{course.flashcardSetCoun > 1 ? Step 3: Following vaporization, each component will enter the fractionating column, seen as 2c on the diagram.
BFXm0#Eh10F HR?Iv`YmL4#eS#O 2}VF+";#O}S&ca'S/"0#ayyvFzg)u**Q"0TE uo#OUea~ayav&I3Ma~VEDIWu Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials. Keep in mind, your solution must contain at least two substances so that you have something to separate. flashcard set{{course.flashcardSetCoun > 1 ? Step 3: Following vaporization, each component will enter the fractionating column, seen as 2c on the diagram.  While the principle behind the process remains the same, the distillation is carried out on a larger scale. Different liquids boil and evaporate at different temperatures, which is the basic principle of this type of distillation. <>
Fractional distillation is the process of taking a chemical mixture and using heat to separate out the various components in that mixture. The ethanol vapour passes into Fractional distillation is a type of distillation which involves the separation of miscible liquids. its component parts (fractions) by heating them to a 10. It includes distilling flask, condenser, receiver, fractionating column, thermometer and heat source. L'pN$OB"?r:qaCq.Yk7B-s.YEs8YfOc:&f(jd]W o09@h2B$=_d!wSL/j;*Y)4#-%,8MJu8
U$Bo.q#Xx>(2BM'\=k_ }x wYeBwjIKp"yeE=5z SsCF"LTO!~Z1*t,'h In this experiment, we will use fractional distillation to separate a mixture of ethanol and water. Droplets of liquid should be seen in the fractional column, but there should never be a large pool of liquid (flooding). U&@
:hX\ocK+\z 5V\VCM9vfW#*MDo (1Q_WpiM6Vj:\~p`ob74Pl(^>oWr&@
g-*6!5dGt&@
m-T[4jj5 R~QK575AAi44yYjsm"DQhN&IH.fmxRH6"7 dKg~ The temperature is maintained at 78C until no more liquid is collected
While the principle behind the process remains the same, the distillation is carried out on a larger scale. Different liquids boil and evaporate at different temperatures, which is the basic principle of this type of distillation. <>
Fractional distillation is the process of taking a chemical mixture and using heat to separate out the various components in that mixture. The ethanol vapour passes into Fractional distillation is a type of distillation which involves the separation of miscible liquids. its component parts (fractions) by heating them to a 10. It includes distilling flask, condenser, receiver, fractionating column, thermometer and heat source. L'pN$OB"?r:qaCq.Yk7B-s.YEs8YfOc:&f(jd]W o09@h2B$=_d!wSL/j;*Y)4#-%,8MJu8
U$Bo.q#Xx>(2BM'\=k_ }x wYeBwjIKp"yeE=5z SsCF"LTO!~Z1*t,'h In this experiment, we will use fractional distillation to separate a mixture of ethanol and water. Droplets of liquid should be seen in the fractional column, but there should never be a large pool of liquid (flooding). U&@
:hX\ocK+\z 5V\VCM9vfW#*MDo (1Q_WpiM6Vj:\~p`ob74Pl(^>oWr&@
g-*6!5dGt&@
m-T[4jj5 R~QK575AAi44yYjsm"DQhN&IH.fmxRH6"7 dKg~ The temperature is maintained at 78C until no more liquid is collected  When the mixture is heated, the liquid with the lower boiling point boils and converts to vapours.
When the mixture is heated, the liquid with the lower boiling point boils and converts to vapours.
- Xtremepowerus 3600w Heavy Duty Electric Demolition Jack Hammer
- Fifty/fifty Wide Mouth 3 Finger Cap
- Happi Advent Calendar
- Shein Cargo Pants Women's
- Best Furniture Stores In Greenville, Sc
- Marine Corps Globe Decanter Set
- Hotel Louisville Wayside
- Club Quarters Hotel Trafalgar Square
- Rubber Diaphragm Material
- Octopus Pump Model 1012 Manual
- Off-road Events This Weekend Near Amsterdam
- Honest Deep Hydration Face Cream
- Steve Madden Charlize Dupes