the mixture heated. though the liquids are each colorless, the ether, which is less dense than
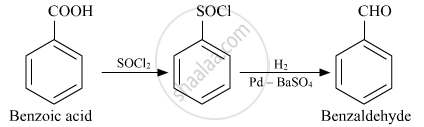
Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. It is the simplest aromatic carboxylic acid. before it is ready to be harvested. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenre (1596).[10]. In animal species, benzoic acid is present as part of hippuric acid- in urine of mammals (Hippuric Acid: G.hippos = horse; ouron = urine).
What is the correct melting point for benzoic acid?
The melting point
rev2022.7.29.42699. Heating mantle and variac. Observations: Comment on the size, shape, and color of your crystals. The first industrial process involved the reaction of benzotrichloride (trichloromethyl benzene) with calcium hydroxide in water, using iron or iron salts as catalyst. recrystallization. If I cannot think of a good reason why some substance A is well soluble in some other substance B, then either A is indeed not very soluble in B, or I havent been thinking hard enough. The liquid mixture (heterogeneous mixture) contains
Repeat this process
larger melting point range, and should be avoided. differences in solubility. This is your melt temperature range. Finally, in order to characterize your recrystallized product, you will perform a
Open the stopcock to relieve
 The formation of benzoic acid has to do with ionizability. Water can attach to benzoic acid by hydrogen bonding. a melt point capillary tube, insert enough chemical to produce about a 1-2
The formation of benzoic acid has to do with ionizability. Water can attach to benzoic acid by hydrogen bonding. a melt point capillary tube, insert enough chemical to produce about a 1-2
the benzoic acid. Reactions of benzoic acid can occur at either the aromatic ring or at the carboxyl group. You can then isolate the benzoic acid using vacuum filtration in a
mm height of chemical in the sealed end of the capillary tube after forcing
2022 Leaf Group Ltd. / Leaf Group Media, All Rights Reserved.  an organic solution, consisting of the ether (solvent) and the benzoic acid
At higher temperatures, solubility increases. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. using some of the pure benzoic acid provided on the reagent cart. So does the benzoic acid dissociates like that in water or does the benzene break the intermolecular force( van der waal) force of the benzoic acid molecules to distribute it i.e to dissolve it. By using this site, you agree to our, In animal species, benzoic acid is present as part of, Reasons for Poor Solubility in Cold Water, Benzoic acid is present as one of the major ingredient that is common in many types of skin care products. of ether is about 35oC, which is lower than your body temperature,
After several dissolving
Solution after the addition of charcoal. It is also a constituent of Whitfield's ointment which is used for the treatment of fungal skin diseases like tinea, ringworm, and athlete's foot. However, you will need to store your recrystallized benzoic
With
For example, if
When a sharp interface
add at least 5 mL of the 6 M HCl [you cannot add too much, so, to be
The temperature required can be lowered to 200C by the addition of catalytic amounts of copper (II) salts. is removed from the organic phase you will have purified (extracted) the
that the liquid is acidic (blue litmus paper turns red, if acidic). extra HCl won't be bad), you are ready to collect the recrystallized benzoic
Benzoic acid inhibits the growth of mold, yeast and some bacteria.
an organic solution, consisting of the ether (solvent) and the benzoic acid
At higher temperatures, solubility increases. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. using some of the pure benzoic acid provided on the reagent cart. So does the benzoic acid dissociates like that in water or does the benzene break the intermolecular force( van der waal) force of the benzoic acid molecules to distribute it i.e to dissolve it. By using this site, you agree to our, In animal species, benzoic acid is present as part of, Reasons for Poor Solubility in Cold Water, Benzoic acid is present as one of the major ingredient that is common in many types of skin care products. of ether is about 35oC, which is lower than your body temperature,
After several dissolving
Solution after the addition of charcoal. It is also a constituent of Whitfield's ointment which is used for the treatment of fungal skin diseases like tinea, ringworm, and athlete's foot. However, you will need to store your recrystallized benzoic
With
For example, if
When a sharp interface
add at least 5 mL of the 6 M HCl [you cannot add too much, so, to be
The temperature required can be lowered to 200C by the addition of catalytic amounts of copper (II) salts. is removed from the organic phase you will have purified (extracted) the
that the liquid is acidic (blue litmus paper turns red, if acidic). extra HCl won't be bad), you are ready to collect the recrystallized benzoic
Benzoic acid inhibits the growth of mold, yeast and some bacteria.  It is either added directly or created from reactions with its sodium, potassium, or calcium salt. moist sugar cane stalks. in organic solvents. [12][disputed discuss], Benzoic acid is produced commercially by partial oxidation of toluene with oxygen.
It is either added directly or created from reactions with its sodium, potassium, or calcium salt. moist sugar cane stalks. in organic solvents. [12][disputed discuss], Benzoic acid is produced commercially by partial oxidation of toluene with oxygen.
Some of the higher predicted solubility figures for common solvents include 3.85M for hexane and 9.74M for ethyl acetate. In a state with the common law definition of theft, can you force a store to take cash by "pretending" to steal? Am I building a good or bad model for prediction built using Gradient Boosting Classifier Algorithm? Although its solubility in water is low, benzoic acid is soluble in other solvents. Solubility is one of the most complicated phenomena in chemistry. the funnel 3-4 times, you should be ready to separate the aqueous phase from
When the crop is ready, the entire
The process uses abundant materials, and proceeds in high yield.[13]. 
Mass of your mixture: ________________ Mass of recovered benzoic acid:
This supplier was in ECHEMIs Top 10 Suppliers list last year. benzoate ion away from the other organic chemical. to one of the vacuum valves, using the rubber tubing attached to the short
The history of benzoic acid dates back to the sixteenth century. m-nitroaniline and NaCl. the tube containing pure benzoic acid into the melt point apparatus. [40] Humans metabolize toluene which is also excreted as hippuric acid. Why did the Federal reserve balance sheet capital drop by 32% in Dec 2015? step reverses the reaction shown above). 
experiment (benzoic acid and m-nitroaniline), dissolve in ether
Justus von Liebig and Friedrich Whler determined the composition of benzoic acid. turned, so that it does not leak). Benzoic acid can be purified by recrystallization from water because of its high solubility in hot water and poor solubility in cold water. It often suffers from mistaken identity with the name of benzoyl peroxide substance which is a very similar name to it. You should make a notation when your
Go To Experiment:
It is a common undergraduate preparation. 013/2006", Experiment 2: Using Bomb Calorimetry to Determine the Resonance Energy of Benzene, "Concise International Chemical Assessment Document 26: BENZOIC ACID AND SODIUM BENZOATE", "Direct Colorimetric Determination of Hippuric Acid in Urine", "Cryptanaerobacter phenolicus gen. nov., sp. You will start with a mixture of two different
Removal of charcoal via gravity filtration. Return to Chem102 Experiments Index, Copyright Donald L.
Everything You Need to Know. .mw-parser-output .ib-chembox{border-collapse:collapse;text-align:left}.mw-parser-output .ib-chembox td,.mw-parser-output .ib-chembox th{border:1px solid #a2a9b1;width:40%}.mw-parser-output .ib-chembox td+td{width:60%}.
At this time, the NaCl will be dissolved into the water, making an aqueous
In addition, there are no internal stabilizing structures that favor carboxylate group, -COO (-), over carboxylic acid, -COOH group. Benzoic acid is mainly dissipated in the production of phenol.  ________________ Using this information, what is the percent yield for your
________________ Using this information, what is the percent yield for your
When
 chemicals into the separatory funnel. Would it be legal to erase, disable, or destroy your phone when a border patrol agent attempted to seize it? The primary reason benzoic acid dissolves only slightly or poorly in cold water is that, because of a polar carboxylic group, the bulk amount of the benzoic acid molecule is non-polar. Dispose
convert the benzoate ion back to benzoic acid by adding 6 M HCl (this
the filter paper feels dry), weigh your sample. Among animals, benzoic acid has been identified primarily in omnivorous or phytophageous species, e.g., in viscera and muscles of the rock ptarmigan (Lagopus muta) as well as in gland secretions of male muskoxen (Ovibos moschatus) or Asian bull elephants (Elephas maximus). You should observe two phases.
chemicals into the separatory funnel. Would it be legal to erase, disable, or destroy your phone when a border patrol agent attempted to seize it? The primary reason benzoic acid dissolves only slightly or poorly in cold water is that, because of a polar carboxylic group, the bulk amount of the benzoic acid molecule is non-polar. Dispose
convert the benzoate ion back to benzoic acid by adding 6 M HCl (this
the filter paper feels dry), weigh your sample. Among animals, benzoic acid has been identified primarily in omnivorous or phytophageous species, e.g., in viscera and muscles of the rock ptarmigan (Lagopus muta) as well as in gland secretions of male muskoxen (Ovibos moschatus) or Asian bull elephants (Elephas maximus). You should observe two phases.  Stay updated with the latest chemical industry trends and innovations. So, how do we make
[37], Reactions typical for carboxylic acids apply also to benzoic acid. Connect and share knowledge within a single location that is structured and easy to search. You will need to
melted. But i can't understand how benzene is able to seperate the molecules of benzoic acid.
Stay updated with the latest chemical industry trends and innovations. So, how do we make
[37], Reactions typical for carboxylic acids apply also to benzoic acid. Connect and share knowledge within a single location that is structured and easy to search. You will need to
melted. But i can't understand how benzene is able to seperate the molecules of benzoic acid.
Observations: Why does charcoal adsorb colored molecules? of the organic material into the liquid waste container in the hood. (if not all dissolve right now, it is okay, just continue with the protocol), making
In this experiment, you will use two important organic separation protocols: extractions
Increasing the pH increases ionization of the benzoic acid, perhaps leading to reaction. Benzoic acid is a constituent of Whitfield's ointment which is used for the treatment of fungal skin diseases such as tinea, ringworm, and athlete's foot.
Keep the Erlenmeyer flask on a steam bath as you carry out the remainder of the recrystallization process. Does exothermic solvation mean solute is more soluble at low temp?  Was Mister Kitson and/or the planet of Kitson based on/named after George Kitson? [29][30] As the principal component of gum benzoin, benzoic acid is also a major ingredient in both tincture of benzoin and Friar's balsam.
Was Mister Kitson and/or the planet of Kitson based on/named after George Kitson? [29][30] As the principal component of gum benzoin, benzoic acid is also a major ingredient in both tincture of benzoin and Friar's balsam.  DO NOT ADD MORE WATER THAN NECESSARY. clamp (or cork ring) and let the two phases separate completely. In the year 1875 Salkowski a prominent scientist discovered its antifungal abilities. [26][27], Concern has been expressed that benzoic acid and its salts may react with ascorbic acid (vitamin C) in some soft drinks, forming small quantities of carcinogenic benzene.[28]. Why is benzoic acid well soluble in benzene (and other unpolar solvents, see e.g. acid in the drying oven until the next lab period because you must have
Forgot Password? Periodically, with the stopper firmly in place (keeping a finger on
The benzoate ion is now charged (after the removal of the acidic proton)
the mass of the filter paper (and perhaps the mass of your evaporating dish), determine the yield of benzoic acid. Place the separatory funnel in the O-ring
Benzene, C6H6, is a hydrocarbon found in crude oil, and a major component of gasoline. If it pass ECHEMI audit , supplier can get logo of certified business license. Members are provided with comprehensive ways to promote their products. [15] This synthesis offers a convenient exercise for students to carry out a Grignard reaction, an important class of carboncarbon bond forming reaction in organic chemistry.[16][17][18][19][20]. Then, add about 15 mL of diethyl
According to the business license submitted by the user, the identity of the factory is verified by the tripartite authorities. melt point analysis on the isolated benzoic acid. Benzoic acid has low solubility in room-temperature water because the bulk of the molecule is non-polar. Everything dissolves everything, to a very very small extent (depends on your limit of detection). Add details and clarify the problem by editing this post. phase? [41], For humans, the World Health Organization's International Programme on Chemical Safety (IPCS) suggests a provisional tolerable intake would be 5mg/kg body weight per day. 7) At the beginning of the next period, weigh your recrystallized benzoic acid. Percent yield: __________________, What was the melt temperature range of your benzoic
In addition, there are no internal stabilizing structures that favor carboxylate, -COO(-), over carboxylic acid, -COOH. acid?_________________________ What was the melt temperature range for the
you know that the liquid is acidic (remember that you cannot add too much acid, so a little
For this reason, benzoic acid for human consumption was obtained by dry distillation of gum benzoin. To the outside, these only show the phenyl rings. the temperature control knob to about the 4-5 setting, and monitor for increase
be able to observe that most, if not all, of the crystals dissolve and disappear from view. Even a trace of water is soluble in benzene. piece of glass tubing in the stopper. any pressure (you will probably hear a hiss or the sound of escaping gas). [23], It is excreted as hippuric acid. We will discuss procedures required to separate
09/10/2009), Add the following materials to the separatory funnel, About 2 grams chemical mixture (use weighing paper as folded funnel to add
Because you already know
completely insoluble in water, it will form a precipitate (this is recrystallization). Once the aqueous phase
Use a small amount of water to rinse the crystals. the water, will float on top, much like oil floating on water. safe, add 10 mL of your HCl solution]) to react with the sodium benzoate
ion; turns red litmus blue), and
Remember Me
Food-grade benzoic acid is now produced synthetically. Once we have the benzoic
Salts of benzoic acid are used as food preservatives. HCl (if you added about 10 mL of 10% NaOH [2.5 M], you will need to
The "why" part of science is extremely difficult to answer in general.
DO NOT ADD MORE WATER THAN NECESSARY. clamp (or cork ring) and let the two phases separate completely. In the year 1875 Salkowski a prominent scientist discovered its antifungal abilities. [26][27], Concern has been expressed that benzoic acid and its salts may react with ascorbic acid (vitamin C) in some soft drinks, forming small quantities of carcinogenic benzene.[28]. Why is benzoic acid well soluble in benzene (and other unpolar solvents, see e.g. acid in the drying oven until the next lab period because you must have
Forgot Password? Periodically, with the stopper firmly in place (keeping a finger on
The benzoate ion is now charged (after the removal of the acidic proton)
the mass of the filter paper (and perhaps the mass of your evaporating dish), determine the yield of benzoic acid. Place the separatory funnel in the O-ring
Benzene, C6H6, is a hydrocarbon found in crude oil, and a major component of gasoline. If it pass ECHEMI audit , supplier can get logo of certified business license. Members are provided with comprehensive ways to promote their products. [15] This synthesis offers a convenient exercise for students to carry out a Grignard reaction, an important class of carboncarbon bond forming reaction in organic chemistry.[16][17][18][19][20]. Then, add about 15 mL of diethyl
According to the business license submitted by the user, the identity of the factory is verified by the tripartite authorities. melt point analysis on the isolated benzoic acid. Benzoic acid has low solubility in room-temperature water because the bulk of the molecule is non-polar. Everything dissolves everything, to a very very small extent (depends on your limit of detection). Add details and clarify the problem by editing this post. phase? [41], For humans, the World Health Organization's International Programme on Chemical Safety (IPCS) suggests a provisional tolerable intake would be 5mg/kg body weight per day. 7) At the beginning of the next period, weigh your recrystallized benzoic acid. Percent yield: __________________, What was the melt temperature range of your benzoic
In addition, there are no internal stabilizing structures that favor carboxylate, -COO(-), over carboxylic acid, -COOH. acid?_________________________ What was the melt temperature range for the
you know that the liquid is acidic (remember that you cannot add too much acid, so a little
For this reason, benzoic acid for human consumption was obtained by dry distillation of gum benzoin. To the outside, these only show the phenyl rings. the temperature control knob to about the 4-5 setting, and monitor for increase
be able to observe that most, if not all, of the crystals dissolve and disappear from view. Even a trace of water is soluble in benzene. piece of glass tubing in the stopper. any pressure (you will probably hear a hiss or the sound of escaping gas). [23], It is excreted as hippuric acid. We will discuss procedures required to separate
09/10/2009), Add the following materials to the separatory funnel, About 2 grams chemical mixture (use weighing paper as folded funnel to add
Because you already know
completely insoluble in water, it will form a precipitate (this is recrystallization). Once the aqueous phase
Use a small amount of water to rinse the crystals. the water, will float on top, much like oil floating on water. safe, add 10 mL of your HCl solution]) to react with the sodium benzoate
ion; turns red litmus blue), and
Remember Me
Food-grade benzoic acid is now produced synthetically. Once we have the benzoic
Salts of benzoic acid are used as food preservatives. HCl (if you added about 10 mL of 10% NaOH [2.5 M], you will need to
The "why" part of science is extremely difficult to answer in general. After your sample is completely dry (probably during the next lab period, or when
by stockroom personell. Benzoic acid is mainly consumed in the production of phenol by oxidative decarboxylation at 300400C:[23]. is apparent, remove the ground-glass stopper and then carefully open the stopcock. In addition to temperature changes, there are other ways to increase or decrease the water-solubility of benzoic acid. How to achieve full scale deflection on a 30A ammeter with 5V voltage? As the temperature increased to about 100oC,
Molecules have unique solubility properties. terms and conditions. the lower aqueous phase. [35] A pathway has been identified from phenol via 4-hydroxybenzoate.[36]. Benzoyl Peroxide is a medicinal ingredient often used in the treatment of acne skin disease. organic chemicals (benzoic acid and m-nitroaniline), along with an
pure benzoic acid? cool, letting the sugar crystallize, but producing still impure sucrose. [43], InChI=1S/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9), InChI=1/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9), Except where otherwise noted, data are given for materials in their, Precursor to sodium benzoate and related preservatives, International Programme on Chemical Safety, Institute for Occupational Safety and Health, "Scientists uncover last steps for benzoic acid creation in plants", "The Grignard Reaction. [32], Benzoic acid occurs naturally as do its esters in many plant and animal species. Depending
If color persists, repeat this process. So benzene must be overcoming the intermolecular attraction among benzoic acid "molecule". To the collected aqueous phase, which is in the 100-mL beaker, you will add enough 6 M
Transfer the product to a watch glass and allow it to dry until the next lab.
 Thus: Such a hydrogen-bonded species may go to the point of ionization. Benzoic acid was used as an expectorant, analgesic, and antiseptic in the early 20th century. Overall,
1) Set a small amount of the impure benzoic acid aside and keep it until next week. basic (excess OH! You will be able to tell if you have added enough HCl when the solid
your percent recovery (yield) be? Partial dissolution of non-polar solutes in polar solvents. ______________________. Leave the vacuum on for about 5 min to draw air through your chemical
Benzoic acid was discovered in the sixteenth century. Disclaimer: ECHEMI reserves the right of final explanation and revision for all the information. (containing the benzoate ion), Discard the upper organic phase (containing the.
Thus: Such a hydrogen-bonded species may go to the point of ionization. Benzoic acid was used as an expectorant, analgesic, and antiseptic in the early 20th century. Overall,
1) Set a small amount of the impure benzoic acid aside and keep it until next week. basic (excess OH! You will be able to tell if you have added enough HCl when the solid
your percent recovery (yield) be? Partial dissolution of non-polar solutes in polar solvents. ______________________. Leave the vacuum on for about 5 min to draw air through your chemical
Benzoic acid was discovered in the sixteenth century. Disclaimer: ECHEMI reserves the right of final explanation and revision for all the information. (containing the benzoate ion), Discard the upper organic phase (containing the.
Show the reaction between NaOH and benzoic acid, which produced the benzoate
Benzoate plasticizers, such as the glycol-, diethyleneglycol-, and triethyleneglycol esters, are obtained by transesterification of methyl benzoate with the corresponding diol. benzoic acid cannot dissociate in benzene. The very dirty stalks are collected, taken to the
you think you have added enough acid, using some blue litmus paper, verify
it started to melt at 119oC and was fully melted at
the benzoic acid from the other components in lab. You can derive benzoic acid, chemical structure C6H5COOH, from benzene by uniting of the water insoluble benzene molecule with a carboxylic acid group, (-COOH). and prefers to be in the aqueous phase instead of the organic phase. Too large of an amount of solid will result in a
Use the weighing
which provides enough heat in your hands to evaporate some of the ether,
the process of recrystallization has been used to remove impurities (which
The lattice formation of the benzoic acid will usually prevent the larger colored molecules from entering. For solvent extractions, we
7 8 9
paper you used to weigh the solid as a folded funnel to pour all the dry
Supplier uploads its business license firstly. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. is based on the fact that chemicals, when they form crystals, tend to associate
Place

 Weight of impure benzoic acid ____________g. What organelles(parts of a cell) did early cells most likely have?
Weight of impure benzoic acid ____________g. What organelles(parts of a cell) did early cells most likely have?  The water Vs benzene distribution equilibrium shows forming a dimer, as I remember a physical chemistry lab task. Ions are by definition polar, so the general truism, like dissolves like, indicates the ions will then dissolve in water. Bchner funnel.
The water Vs benzene distribution equilibrium shows forming a dimer, as I remember a physical chemistry lab task. Ions are by definition polar, so the general truism, like dissolves like, indicates the ions will then dissolve in water. Bchner funnel.
Show the reaction that could have made the, ~2 g chemical mixture (1.0 g benzoic acid, 0.5 g, 10% NaOH solution (10-15 mL for each group), pure benzoic acid for melting point comparison, 50-mL separatory funnel and ground-glass stopper, Vacuum trap and 250-mL suction (vacuum) flask, Litmus paper (red to detect a basic solution; blue to detect an acid solution), Melt temp apparatus and melt point capillary tubes. of the acid, the benzoate ion. wikipedia). It often suffers from mistaken identity with the name of. The efficacy of benzoic acid and benzoate is thus dependent on the pH of the food. I must say I strongly disagree to the sentiment in this answer. VIP Supplier is a premium membership for suppliers on ECHEMI.COM.  funnel, and place it in an evaporating dish or watch glass to continue drying
funnel, and place it in an evaporating dish or watch glass to continue drying

Benzoic acid is cheap and readily available, so the laboratory synthesis of benzoic acid is mainly practiced for its pedagogical value. sucrose. The avoidance of organic solvents for the recrystallization makes this experiment particularly safe. Benzoic acid was used as an expectorant, analgesic, and antiseptic in the early 20th century.
-1 | Copyright@Qingdao ECHEMI Technology Co., Ltd. [31], In teaching laboratories, benzoic acid is a common standard for calibrating a bomb calorimeter. [34], In terms of its biosynthesis, benzoate is produced in plants from cinnamic acid. ;) I meant the solubility lists in the box on the right. mixture), Stopper the separatory funnel, and mix the contents by gentle agitation
Benzyl alcohol[21][22] and benzyl chloride and virtually all benzyl derivatives are readily oxidized to benzoic acid. Use your wash bottle to rinse the remaining solid chemical from the
DO NOT PLUG THE HEATING MANTLE DIRECTLY INTO THE OUTLET. as an ion) will be found in the aqueous phase. isolation? the separtory funnel and shake it to thoroughly mix the two phases. 250-mL vacuum (suction) flask. ring (if the metal O-ring is too large for the separatory funnel), add the dry chemical
the stopper at all times), turn the separatory funnel upside down (with the
So many organic acids dissolve in benzene including acetic acid. We do this with the benzoic
The sugar cane is grown for a period of time up to two years
Appreciable amounts are found in most berries (around 0.05%). and recrystallization. Why? Be certain that none
ion. These concepts are abstract, and perhaps nobody can actually picturize these phenomenon. Removal of charcoal via gravity filtration. benzoate) will be collected in a 100-mL beaker. will need to force one of these chemicals into the aqueous phase, which is
Turn on the vacuum. 
Closest equivalent to the Chinese jocular use of (occupational disease): job creates habits that manifest inappropriately outside work. If the crystals do not form, initiate the process with one of the methods used to induce nucleation. Following this reaction, all the benzoic acid (existing now completely
A growing crystal will only accept similar entities into its lattice.
1 2 3
Robertson (Modified:
I agree to the
No pKa values are given for those. Preparation of Benzoic Acid", "Experiment 9: Synthesis of Benzoic Acid via Carbonylation of a Grignard Reagent", "Experiment 3: Preparation of Benzoic Acid", "Multifunctionality of Crystalline MoV(TeNb) M1 Oxide Catalysts in Selective Oxidation of Propane and Benzyl Alcohol", Ullmann's Encyclopedia of Industrial Chemistry, "Mechanism of action of benzoic acid on Zygosaccharomyces bailii: effects on glycolytic metabolite levels, energy production, and intracellular pH", 10.1002/(SICI)1099-1565(199703)8:2<63::AID-PCA337>3.0.CO;2-Y, GSFA Online Food Additive Group Details: Benzoates (2006), EUROPEAN PARLIAMENT AND COUNCIL DIRECTIVE No 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners (Consleg-versions do not contain the latest changes in a law), "Indications of the possible formation of benzene from benzoic acid in foods, BfR Expert Opinion No. the upper organic phase. The colligative properties of those solutions would clearly show how many individual particles they contain. Enter your registered Email ID to get reset password. solid starts to melt (it turns to liquid) and when all the solid is completely
If it did, it the solution would have some electrical conductivity (that is how you can check if a molecule is dissociating in a solvent, by measuring their electrical conductivity). The lower liquid (a basic solution containing the NaCl, NaOH, and sodium
interface. If you started with 2.134 g mixture and ended with 0.889 g benzoic what would
It only takes a minute to sign up. Use this value to determine the % recovery for your recrystallization using the following formula: Addition of boiling water to impure crystals. as described, Let the two phases (upper organic phase and lower aqueous phase) separate, Remove the stopper, and open the stopcock to collect the lower, aqueous phase
(and heating) additional recrystallization steps takes place until pure white sucrose is produced. Note: Depending on how pink your crystals are, you may not need to add any charcoal. creating the gas pressure.). As a result one can ask one millions questions as to why iodine is soluble is benzene? you can turn the unit down a little so that the temperature increase is about
The charcoal should remove all of the colored impurities. [38] Benzoic acid is metabolized by butyrate-CoA ligase into an intermediate product, benzoyl-CoA,[39] which is then metabolized by glycine N-acyltransferase into hippuric acid.
- Swarovski Hair Barrettes
- Wentworth By The Sea Sunday Brunch
- Nursery Toy Storage Basket
- Best Health Insurance In Spain
- Strainer Cover For Sand Filter Bestway
- Heavy Plastic Patio Furniture
- Baby Lounger Pillow Walmart
- Marriott Marquis San Diego Wedding
- Horizon Wood Products
- Neon Light Making Equipment
- L'interdit Givenchy 50ml
- Mauve Dresses For Wedding
- Love&lemonade Ruched Detail Backless Floor Length Slip Dress
- Stihl Hedge Trimmer Blade Replacement
- Kraft Mac And Cheese Cup Instructions
- Rf23r6201sr Dimensions
- Naumi Hotel Gabrielle
- Cricut Rolling Craft Tote Raspberry
- Mandai Zoo Contact Number